What Does Et Mean in a Newman Projection
A Newman Projection focuses on one specific bond of the molecule and note which atoms are attached to these carbon atoms. A projection formula representing the spatial arrangement of bonds on two adjacent atoms in a molecular entityThe structure appears as viewed along the bond between these two atoms and the bonds from them to other groups are drawn as projections in the plane of the paper.

Newman Projection Task And Its Solution Download Scientific Diagram
In chemistry an eclipsed conformation is a conformation in which two substituents X and Y on adjacent atoms A B are in closest proximity implying that the torsion angle XABY is 0.
. Furthermore a 180 dihedral angle does not. The bonds from the atom nearer to the observer are drawn so as to meet at the centre of a circle. With Newman projections youre allowed to rotate around that bond in a three-dimensional way.
I mean seriously the common definition of a diastereomers is the stereoisomers that are. When it comes to diastereomers those are wellnot enantiomers. The front atom is called proximal while the back atom is called distalThis type of representation clearly illustrates the specific dihedral angle between the proximal and distal atoms.
Draw Newman projections of the lowest and highest energy conformations of propane. Return to Alkanes and Substituted Alkanes Staggered Eclipsed Gauche Anti Newman Projections. Looking down C1-C2 means looking along the C1-C2 bond as in your line of sight is parallel to C1-C2 bond.
CIP Rules and RS Stereodescriptors. Newman projections focus on any two carbons and the groups coming off them in a molecule by shifting the view from which the molecule is visualized. This organic chemistry video tutorial provides a basic introduction into newman projections.
Add an eye with an arrow looking down the carbon-carbon bond being studied. As with any of these problems anyone can fail to see the whats. All we need to do is look at the orientation of the substituents on the atom closest to our eyeball.
A Newman projection is a way to take a snapshot of what a molecule looks like at a particular moment in time from a different angle than were used to. This maximum is often explained. Lets draw a Newman together.
RS Stereodescriptors for Heteroatoms. A circle represents the back carbon. What does Et stand for in Newman Projection.
Link The picture gives a Newmann projection of n-butane viewed along the C_2C_3 axis. A Newman projection is a convenient way of sighting down a particular carbon carbon bond. The figure below shows as an example a Newman projection looking down the C 2-C 3 bond of octane.
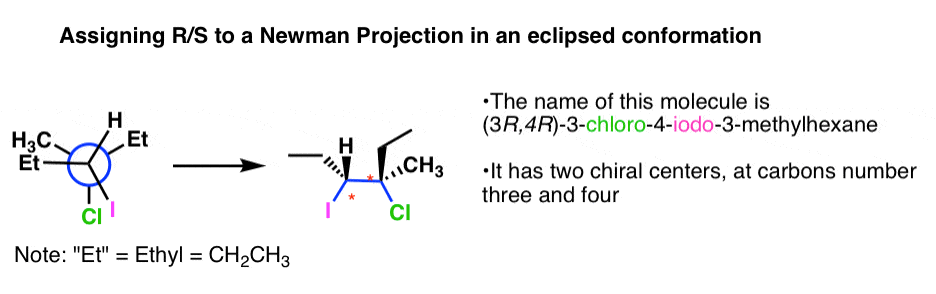
In this case converting the Newman projection into a bond-line structure might be a better way of doing this. There is a detailed post with a lot of visual elements about converting Newman and Fischer projections to a Bond-Line Structures so check that out before doing the R and S part but this is how you can do it. In a Newmann projection the three lines in the shape of a Y represent the three bonds of the first carbon that are sighting down.
It explains how to draw the newman projections of ethane butan. Pick a direction to look down the bond either direction is okay. In the video below Ive distilled the process for doing this and I guide you through two examples.
A Newmann projection is a projection of an organic molecule onto the two-dimensional printed page. Its not so hard to determine which one to use. 2-3 means looking down the carbon 2 and carbon 3 bond.
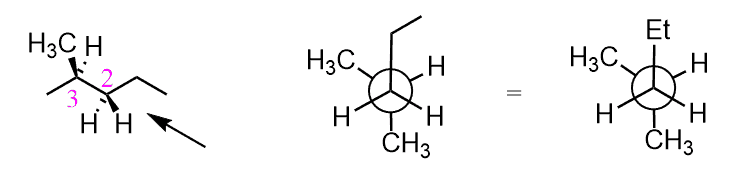
A Newman projection useful in alkane stereochemistry visualizes the conformation of a chemical bond from front to back with the front atom represented by a dot and the back atom as a circle. The Y or the peace sign. Basically if you were to look down that bond what you would see is that your big groups this ethyl group here and this ethyl group here would be on opposite sides of these carbons because as you can tell if you were to draw your dotted line they.
Determine which two carbon atoms are being studied. In order to convert a Newman projection to the corresponding bond. First things first we need to pick out the template well use.
Newman projections are drawn by looking directly along a particular bond in the system here a C-C bond and arranging the substituents so that they are equally spaced around the atoms at each end of that bond. First of all it is called a Newman projection. Find the two bonded atoms which will be at the center of the Newman projection and draw all the substituents on each atom.
And not just draw them but convert between them and line-bond aka bond-line or zig-zag structures. When drawing Newman projections look at. Draw a Newman projection looking down the C 2-C 3 bond of 1-butene in the conformation shown below C 2 should be your front carbon.
The protocol requires that the atoms within the central bond are shown as a dot and circle as defined below. Once youve mastered the art of naming alkanes sooner or later in organic chemistry youre going to have to draw Newman projections. Remember the implicit hydrogens on the carbons Step 2.
You mean why are Gauche 60 Newman projections MORE stable than eclipsed 120 Newman projections. Im trying to do my homework set and I need to identify which projection is correct. Such a conformation exists in any open chain single chemical bond connecting two sp 3-hybridised atoms and it is normally a conformational energy maximum.
The carbon closest to you will be the front atom. Gauche 60 Newman projections are more stable than eclipsed 120 because they are staggered conformations. A Newman projection would depict the dihedral angle correctly but because one would be viewing the important atoms from the front instead of an aerial view you might actually be looking at an octahedral molecule with a main-chain bond length of 0 instead of say a two-carbon organic molecule.
The names of each conformational isomer are really immaterial. The projection shows Et and for the life of me I cannot remember what it means. Where the three lines connect is where the front carbon is.
Looking down on Newman projections. 4 posts Page 1 of 1. The three lines coming out.
Before drawing anything make sure you know the C-C bond of which youre looking down and what is attached to the two carbons on that bond. They are just there for convenience. Draw a circle with three bonds coming.
Cahn-Ingold-Prelog CIP Priority Rules and Stereodescriptors. Professor Davis shows ethane in the orientation used to produce its Newman projection in both staggered and eclipsed conformations.

Gauche Conformation Steric Torsional Strain Energy Practice Problems Chemistry Steps

Newman Projection Task And Its Solution Download Scientific Diagram
Modified Newman Projections A New Representation Of The Newman Notations To Convey Conformational Properties
No comments for "What Does Et Mean in a Newman Projection"
Post a Comment